Diagnostic Chemical Processes Test
Next
0 of 11 questions completed
Questions:
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
Information
This sampler diagnostic test is comprised of 11 items, which must be completed within 9 minutes.
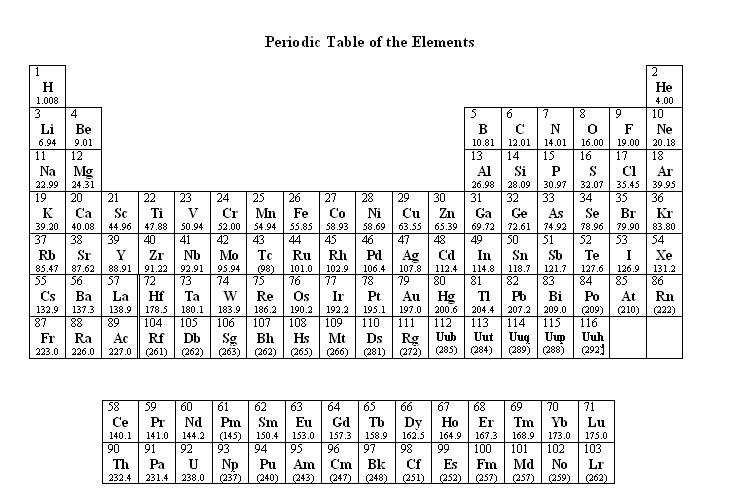
Some items refer to the Periodic Table of Elements. To view the table while in an item screen, click the Exhibit button, located in the lower left corner of the screen. Each test item is a question or incomplete statement followed by suggested answers or completions. Read the item, decide which choice is best, and select the answer with the mouse. A navigation bar is provided, enabling you to skip between questions easily and mark questions for review. |
You have already completed the quiz before. Hence you can not start it again.
Exam is loading ...
You must sign in or sign up to start the exam.
You have to finish following exam, to start this exam:
Results
0 of 11 questions answered correctly
Your time:
Time has elapsed
You answered 0 of 0 (0) questions correct
| Average score |
|
| Your score |
|
Categories
- Chemical Processes 0%
-
Estimated PCAT Chemical Processes Score: 200
Just know, when you truly want success, you’ll never give up on it. No matter how bad the situation may get. Keep your head up and keep on fighting! -
Estimated PCAT Chemical Processes Score: Less than 300
Just know, when you truly want success, you’ll never give up on it. No matter how bad the situation may get. Keep your head up and keep on fighting! -
Estimated PCAT Chemical Processes Score: Less than 330
You’re on the right track. Take your time to reflect on your performance and how you can improve your scores the next time around. Carefully review these solutions, learn from your mistakes and understand the intricacies of each question. You’re going in the correct direction and you’ll only go up from here! -
Estimated PCAT Chemical Processes Score: 340
You’re on the right track. Take your time to reflect on your performance and how you can improve your scores the next time around. Carefully review these solutions, learn from your mistakes and understand the intricacies of each question. You’re going in the correct direction and you’ll only go up from here! -
Estimated PCAT Chemical Processes Score: 370
You’re on the right track. Take your time to reflect on your performance and how you can improve your scores the next time around. Carefully review these solutions, learn from your mistakes and understand the intricacies of each question. You’re going in the correct direction and you’ll only go up from here! -
Estimated PCAT Chemical Processes Score: 390
You’re doing a good job! Keep working on it and you’ll soon see your score in the 20’s. Take your time in understanding your mistakes and in carefully reviewing these solutions and learning from the intricacies of each question. -
Estimated PCAT Chemical Processes Score: 410
Good going! You are really getting to where you need to be. Keep it going! Take your time in understanding your mistakes and in carefully reviewing these solutions and understanding the intricacies of each question. Your goal should be to beat your 410 on the next test! Every point you get correct will get you closer to the perfect 600! -
Estimated PCAT Chemical Processes Score: 410
Good going! You are really getting to where you need to be. Keep it going! Take your time in understanding your mistakes and in carefully reviewing these solutions and understanding the intricacies of each question. Your goal should be to beat your 410 on the next test! Every point you get correct will get you closer to the perfect 600! -
Estimated PCAT Chemical Processes Score: 450
Awesome job! Keep it up and you’ll soon be in the 500’s. Learn from your mistakes and strategize on how you’ll beat your 450! -
Estimated PCAT Chemical Processes Score: 470
Awesome job! You did it! You really outdid yourself today. What can we do differently on the next exam to get yourself up to 500? Lets do it! -
Estimated PCAT Chemical Processes Score: 500
Impressive! You hit 500! Now let’s push you up to the perfect 600! -
Estimated PCAT Chemical Processes Score: 540
You rocked it! That was quite an accomplishment! -
Estimated PCAT Chemical Processes Score: 570 or higher
You are a rockstar! We tip our hats to you!
-
Question 1 of 11
1. Question
The point at which a solid and liquid are in equilibrium and their vapor pressures are equal is referred to as the
Exhibit
-
Question 2 of 11
2. Question
At a pressure of 700 mmHg, a sample of a gas has a volume of 375 mL. If the volume is reduced at a constant temperature to 187.5 mL, what is the final pressure of the gas?
Exhibit

-
Question 3 of 11
3. Question

Exhibit

-
Question 4 of 11
4. Question
Urease
Urease is a polymer with two subunits that are twenty-one and sixty-five kilodaltons in size. The protein is characterized by a highly mobile helix turn helix motif. This motif which is located in the alpha subunit is referred to as a flap that adopts two different conformations. The flap opens up to allow the enzyme’s substrate to enter the active site where hydrolysis occurs. In the closed conformation, the active site is not accessible. In its inactive form, urea exists as an apoenzyme and its activation is dependent on the proteins that shuttle nickel ions into the cell for incorporation in the enzyme’s active site.

Fourier Transform Infrared (FTIR) spectroscopy is an analytic method that is based on the measurement of the molecular vibrations energy of functional groups in organic compounds. FTIR allows uninterrupted monitoring of an enzymatic reaction by a simultaneous observation of the compounds involved in the reaction. To examine continuous urease reactivity, researchers used this method to analyze the respective disappearance and appearance of reactant and product in a reaction catalyzed by a urease protein extracted from a fungus. The results are shown in Figure 1.

Figure 1. FTIR spectra of the substrate (urea) and product (NaHCO3) in a reaction mixture containing 0.4 µg urease. Spectra of the reaction mixture were recorded at several time intervals: 0, 10, 20 and 35 min, as indicated.
Which of the following represents a balanced equation for urea hydrolysis?
Exhibit

-
Question 5 of 11
5. Question
Urease
Urease is a polymer with two subunits that are twenty-one and sixty-five kilodaltons in size. The protein is characterized by a highly mobile helix turn helix motif. This motif which is located in the alpha subunit is referred to as a flap that adopts two different conformations. The flap opens up to allow the enzyme’s substrate to enter the active site where hydrolysis occurs. In the closed conformation, the active site is not accessible. In its inactive form, urea exists as an apoenzyme and its activation is dependent on the proteins that shuttle nickel ions into the cell for incorporation in the enzyme’s active site.

Fourier Transform Infrared (FTIR) spectroscopy is an analytic method that is based on the measurement of the molecular vibrations energy of functional groups in organic compounds. FTIR allows uninterrupted monitoring of an enzymatic reaction by a simultaneous observation of the compounds involved in the reaction. To examine continuous urease reactivity, researchers used this method to analyze the respective disappearance and appearance of reactant and product in a reaction catalyzed by a urease protein extracted from a fungus. The results are shown in Figure 1.

Figure 1. FTIR spectra of the substrate (urea) and product (NaHCO3) in a reaction mixture containing 0.4 µg urease. Spectra of the reaction mixture were recorded at several time intervals: 0, 10, 20 and 35 min, as indicated.
The researchers were able to use FTIR spectroscopy to analyze urease activity because
Exhibit

-
Question 6 of 11
6. Question
Urease
Urease is a polymer with two subunits that are twenty-one and sixty-five kilodaltons in size. The protein is characterized by a highly mobile helix turn helix motif. This motif which is located in the alpha subunit is referred to as a flap that adopts two different conformations. The flap opens up to allow the enzyme’s substrate to enter the active site where hydrolysis occurs. In the closed conformation, the active site is not accessible. In its inactive form, urea exists as an apoenzyme and its activation is dependent on the proteins that shuttle nickel ions into the cell for incorporation in the enzyme’s active site.

Fourier Transform Infrared (FTIR) spectroscopy is an analytic method that is based on the measurement of the molecular vibrations energy of functional groups in organic compounds. FTIR allows uninterrupted monitoring of an enzymatic reaction by a simultaneous observation of the compounds involved in the reaction. To examine continuous urease reactivity, researchers used this method to analyze the respective disappearance and appearance of reactant and product in a reaction catalyzed by a urease protein extracted from a fungus. The results are shown in Figure 1.

Figure 1. FTIR spectra of the substrate (urea) and product (NaHCO3) in a reaction mixture containing 0.4 µg urease. Spectra of the reaction mixture were recorded at several time intervals: 0, 10, 20 and 35 min, as indicated.
The protonation of the ammonia in the urea hydrolysis reaction shown in Reaction 1, which was carried out in water, leads to which of the following?
Exhibit

-
Question 7 of 11
7. Question
Urease
Urease is a polymer with two subunits that are twenty-one and sixty-five kilodaltons in size. The protein is characterized by a highly mobile helix turn helix motif. This motif which is located in the alpha subunit is referred to as a flap that adopts two different conformations. The flap opens up to allow the enzyme’s substrate to enter the active site where hydrolysis occurs. In the closed conformation, the active site is not accessible. In its inactive form, urea exists as an apoenzyme and its activation is dependent on the proteins that shuttle nickel ions into the cell for incorporation in the enzyme’s active site.

Fourier Transform Infrared (FTIR) spectroscopy is an analytic method that is based on the measurement of the molecular vibrations energy of functional groups in organic compounds. FTIR allows uninterrupted monitoring of an enzymatic reaction by a simultaneous observation of the compounds involved in the reaction. To examine continuous urease reactivity, researchers used this method to analyze the respective disappearance and appearance of reactant and product in a reaction catalyzed by a urease protein extracted from a fungus. The results are shown in Figure 1.

Figure 1. FTIR spectra of the substrate (urea) and product (NaHCO3) in a reaction mixture containing 0.4 µg urease. Spectra of the reaction mixture were recorded at several time intervals: 0, 10, 20 and 35 min, as indicated.
Which of the following statements is true?
Exhibit

-
Question 8 of 11
8. Question
Which of the following correctly depicts the quantum numbers for the valence shell of lithium?
Exhibit

-
Question 9 of 11
9. Question

Exhibit

-
Question 10 of 11
10. Question
A compound has a molecular weight of 180.00 g/mol and its empirical formula is CH2O.
What is the molecular formula of the compound?Exhibit

-
Question 11 of 11
11. Question
All of the following compounds react with CH3CH2CH2MgBr except:
Exhibit

- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- Answered
- Review











